Deep Drawing
Introduction:
A process in which a punch forces a
flat sheet metal blank into a die cavity is called a deep drawing. Through this
process we can also produce shallow or moderate depth components parts. It is
important metal working process because it is widely used in manufacturing. Normally, this process is useful in producing
deep parts with relatively simple shapes, with high production rate. There are
many parts made by this technique like pots, pans, all the types of containers
for food and beverages. The tooling and equipment cost is relatively high.
This sheet metal forming process
designed to produce hollow shells, was developed in the mid-19th century. The
company started producing porcelain-enamel-covered iron cookware between 1863
and 1870, one of the first U.S. companies to do so.
Operations:
Drawing of a cup-shaped part is the
basic drawing operation, with dimensions and parameters as pictured in Figure 1.
A blank of diameter Db is drawn into a die cavity by means of a
punch with diameter Dp. The punch and die must have corner radii,
given by Rp and Rd. If the punch and die were to have
sharp corners (Rp and Rd = 0), a hole-punching operation
(and not a very good one) would be accomplished rather than a drawing
operation. The sides of the punch and die are separated by a clearance c. This
clearance in drawing is about 10% greater than the stock thickness:
C = 1.1*t
The punch applies a downward force
F to accomplish the deformation of the metal, and a downward holding force Fh
is applied by the blank holder, as shown in the sketch.
As the punch proceeds downward
toward its final bottom position, the work experiences a complex sequence of
stresses and strains as it is gradually formed into the shape defined by the
punch and die cavity. The stages in the deformation process are illustrated in
Figure 2. As the punch first begins to push into the work, the metal is
subjected to a bending operation. The sheet is simply bent over the corner of
the punch and the corner of the die, as in Figure 2[2].
FIGURE
1 (a) Drawing of a cup shaped part: (1) start of operation before punch
contacts work, and (2) near end of stroke; and (b) corresponding work part: (1)
starting blank, and (2) drawn part. Symbols: C = clearance, Db = blank
diameter, Dp = punch diameter, Rd = die corner radius, Rp
= punch corner radius, F = drawing force, Fh = holding force.
Deep Drawability:
In a deep drawing
operation, failure generally results from the thinning of the cup wall under
high longitudinal tensile stresses. If we follow the movement of the material
as it flows into the die cavity, it can be seen that the sheet metal
(a)
Must be capable of undergoing a reduction in
width due to a reduction in diameter.
(b)
Must also resist thinning under the longitudinal
tensile stresses in the cup wall.
It
has been observed that materials with outstanding deep drawability behave anisotropically.
Plastic deformation in the surface is much more pronounced than in the
thickness. The lankford coefficient (r) is a specific material property
indicating the ratio between width deformation and thickness deformation in the
uniaxial tensile test. Materials with very good deep drawability have an r
value of 2 or below.
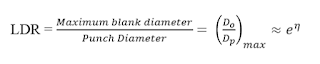
Limiting Drawing Ratio (LDR):
Deep drawability generally is
expressed by the limiting drawing ratio (LDR) and the drawability of a metal is
measured by the ratio of the initial blank diameter to the diameter of the cup
drawn from the blank (usually approximated by the punch diameter).
The theoretical upper limit on LDR is
Where ![]() is an efficiency term
to account for frictional losses. If
is an efficiency term
to account for frictional losses. If ![]() = 1, then LDR = 2.7,
while if
= 1, then LDR = 2.7,
while if ![]() = 0.7, LDR = 2. This
agrees with experience that even with ductile metals it is difficult to draw a
cup with a height much greater than its diameter [1].
= 0.7, LDR = 2. This
agrees with experience that even with ductile metals it is difficult to draw a
cup with a height much greater than its diameter [1].
So for a given material the
limiting draw ratio (LDR), represents the largest blank that can be drawn
through a die Dp without tearing.
Some of the practical
considerations which affect drawability are[1]:
·
Die
radius - should be about 10 times sheet thickness.
·
Punch
radius - sharp radius leads to local thinning and tearing.
·
Clearance
between punch and die ~20 to 40 percent greater than the sheet thickness.
·
Hold-down
pressures about 2 per cent of average of So and Su.
·
Lubricate
die side to reduce friction in drawing.
In order to have successful
drawing of cup shaped part has been found to be a function of the normal
anisotropy, R (also called plastic anisotropy), of the sheet metal.
The effect of planar anisotropy on LDR:
Due to rolling of sheet, grains elongate
into specific directions as shown in Fig. 3, so show different mechanical
properties in different directions, which is called anisotropy.
Figure 3. Rolling produce smaller and elongated grains.
The planar anisotropy of the
sheet is indicated by ![]() R. It is defined in terms of directional
R. It is defined in terms of directional ![]() R,
R,
The planar anisotropy decrease
the LDR value, which in result cause earing defect. In deep drawing, earing
defect is the formation of wavy cup of edges as shown in Fig. 4. They are objectionable
on deep drawn cups because they have to be trimmed off, as they serve no useful
purpose and interfere with further processing of cup, resulting in scrap.
Figure 3. Earing produced due to planar anisotropy.
The value of the strain hardening exponent (n) lies between 0 and 1. Most
metals have an n value between 0.10 and 0.50. What is this value representing?
The response of a metal to cold working can be quantified
by the strain-hardening exponent n. The relationship between true stress 𝞂,
true strain ε, and the strain-hardening exponent n
is governed by so-called power law behavior according to
𝞂 = K*εn
The constant K
(known as the strength coefficient) is equal to the stress when ε = 1. The
value of the strain hardening exponent (n) lies between 0 and 1. Most metals
have an n value between 0.10 and 0.50 [3].
It is actually a measure of the
ability of a metal to strain harden; the larger its magnitude, the greater is
the strain hardening for a given amount of plastic strain [4]. The value of 0 means there is no strain
hardening which indicates that it is perfectly plastic solid, greater than 0
means a little strain hardening which most of the metals shows, while value of
1 indicates that true stress and true strain are linearly dependent which
indicates that it is 100% elastic solid.
The effect of grain-size of sheet metal on the surface finish of the cup:
Grain size of sheet depend on
many factors, rolling temperature, composition and processing rote. In general,
large grain size of sheet in deep drawing operation cause orange peel effect. Orange
peel is a cosmetic defect associated with a rough surface appearance after
forming a component from sheet metal. It is called orange peel because the
surface has the appearance of the surface of an orange. But smaller grain size
results in smoother surface finish.
The effect of “too high” & “too low” blank holder force on deep
drawing:
The drawing force required to perform a given operation can be estimated roughly by the formula [1]:
Where P = total
punch load, 𝞂o=
average flow stress, Dp = punch diameter, Do = blank
diameter, H = hold-down force, B = force required to bend and restraighten
blank, h = wall-thickness, µ = coefficient of friction
If it is too
small, wrinkling occurs. If it is too large, it prevents the metal from flowing
properly toward the die cavity, resulting in stretching and possible tearing of
the sheet metal. Determining the proper holding force involves a delicate
balance between these opposing factors [2].
The purpose of the punch and die radius (𝑅𝑝, 𝑅𝐷)
and the clearance between them:
One of the measures of the
severity of a deep drawing operation is the drawing ratio DR. This is most
easily defined for a cylindrical shape as the ratio of blank diameter Db
to punch diameter Dp. In equation form,
DR = Db/
The drawing ratio provides an
indication, albeit a crude one, of the severity of a given drawing operation.
The greater the ratio, the more severe the operation. An approximate upper
limit on the drawing ratio is a value of 2.0. The actual limiting value for a
given operation depends on punch and die corner radii (Rp and Rd),
friction conditions, depth of draw, and characteristics of the sheet metal
(e.g., ductility, degree of directionality of strength properties in the
metal)[2].
As discussed earlier the clearance
between punch and die ~20 to 40 percent greater than the sheet thickness [1].
Role of Lubrication
Correct lubrication of the sheet
metal is essential if friction, wear, and galling are to be held to the lowest
possible levels during deep drawing. In fact, deep drawing is impossible if the
sheet metal is not lubricated. In actual practice, die materials are selected
after trials using one or more candidate production lubricants. If excessive
wear or galling occurs, a better lubricant is usually applied. For extremely
difficult draws, the best lubricants are usually applied at the outset [5].
References
[1] Dieter, George Ellwood, and D. J.
Bacon. 1988. Mechanical
metallurgy. London: McGraw-Hill.
[2] Groover, Mikell
P., 1939-. Fundamentals of Modern Manufacturing: Materials, Processes, and
Systems. Hoboken, NJ:J. Wiley & Sons, 2007.
[3] Askeland,
Donald R. 1984. The science and
engineering of materials. Monterey, CA: Brooks/Cole Engineering
Division.
[4] Callister, William D., and David G.
Rethwisch. 2008. Fundamentals of
materials science and engineering: an integrated approach. Hoboken, NJ:
John Wiley & Sons.
[5] ASM International. 2006. ASM Handbook. Volume 14B, Volume 14B.
https://doi.org/10.31399/asm.hb.v14b.9781627081863.