Argon Oxygen Decarburization (AOD)
Argon oxygen decarburization offers
improved metal cleanliness, which is measured by low unwanted residual element
contents and gas contents; this ensures superior mechanical properties. The AOD
process is duplexed, with molten metal supplied from a separate melting source
to the AOD refining unit (vessel). The source of the molten metal is usually an
electric arc furnace or a coreless induction furnace. Foundries and integrated
steel mills utilize vessels ranging in nominal capacity from 1 to 160 Mg (1 to
175 tons).
Although the process was initially
targeted for stainless steel production, argon oxygen decarburization is used
in refining a wide range of alloys, including:
v
Stainless steels
v
Tool steels
v
Silicon (electrical) steels
v
Carbon steels, low-alloy steels, and
high-strength low-alloy steels
v
High-temperature alloys and superalloys
Fundamentals
In the AOD process, oxygen, argon,
and nitrogen are injected into a molten metal bath through submerged,
side-mounted tuyeres. The primary aspect of the AOD process is the shift in the
decarburization thermodynamics that is afforded by blowing with mixtures of
oxygen and inert gas as opposed to pure oxygen.
To understand the AOD process, it
is necessary to examine the thermodynamics governing the reactions that occur
in the refining of stainless steel, that is, the relationship among carbon,
chromium, chromium oxide (Cr3O4), and carbon monoxide (CO). The overall
reaction in the decarburization of chromium-containing steel can be written as:
The equilibrium constant, K, is given by:
At a given temperature, there is a
fixed, limited amount of chromium that can exist in the molten bath that is in equilibrium
with carbon. By examining Eq 2, one can see that by reducing the partial
pressure of CO, the quantity of chromium that can exist in the molten bath in
equilibrium with carbon increases. The partial pressure of CO can be reduced by
injecting mixtures of oxygen and inert gas during the decarburization of
stainless steel. Figure 1 illustrates the relationship among carbon, chromium,
and temperature for a partial pressure of CO equal to 1 and 0.10 atm (1000 and 100
mbar, or 760 and 76 torr). The data shows that diluting the partial pressure of
CO allows lower carbon levels to be obtained at higher chromium contents with
lower temperatures.
Fig. 1 Carbon-chromium equilibrium curves.
In refining stainless steel, it is
generally necessary to decarburize the molten bath to less than 0.05% C.
Chromium is quite susceptible to oxidation; therefore, prior to the
introduction of the AOD process, decarburization was accomplished by
withholding most of the chromium until the bath had been decarburized by oxygen
lancing. After the bath was fully decarburized, low-carbon ferrochromium and
other low-carbon ferroalloys were added to the melt to meet chemical
specifications.
Dilution of the partial pressure of
CO allows the removal of carbon to low levels without excessive chromium
oxidation. This practice enables the use of high-carbon ferroalloys in the
charge mix, avoiding the substantially more expensive low carbon ferroalloys.
Fig.
2 Composition changes in refining type 304-L stainless steel using electric
arc furnace practice and argon oxygen decarburization
Equipment
The processing vessel consists of a
refractory-lined steel shell mounted on a tiltable trunnion ring (Fig. 2). With
a removable, conical cover in place, the vessel outline is sometimes described
as pear shaped. Several basic refractory types and various quality levels of
the refractories have gained widespread acceptance (Ref 4, 5). Dolomite
refractories are used in most AOD installations; magnesite chromium
refractories are predominant in small (<9 Mg, or 10 ton) installations.
Fig.
2 Schematic of argon oxygen decarburization vessel.
Processing
1.
Stainless Steels
Charge materials (scrap and
ferroalloys) are melted in the melting furnace. The charge is usually melted
with the chromium, nickel, and manganese concentrations at midrange
specifications. The carbon content at meltdown can vary from 0.50 to 3.0%,
depending on the scrap content of the charge. Once the charge is melted down,
the heat is tapped, and the slag is removed and weighed prior to charging the
AOD vessel.
In the refining of stainless steel
grades, oxygen and inert gas are injected into the bath in a stepwise manner.
The ratio of oxygen to inert gas injected decreases (3:1, 1:1, 1:3) as the
carbon level decreases. Once the aim carbon level is obtained, a reduction mix
(silicon, aluminum, and lime) is added. If extra-low sulfur levels are desired,
a second desulfurization can be added. Both of these steps are followed by an
argon stir. After reduction, a complete chemistry sample is usually taken and
trim additions made following analysis.
Carbon and Low-Alloy Steels
The refining of carbon and
low-alloy steels involves a two-step practice: a carbon removal step, followed
by a reduction/heating step. The lower alloy content of these steels eliminates
the need for injecting less than a 3:1 ratio of oxygen to inert gas. Once the
aim carbon level is obtained, carbon steels are processed similarly to
stainless steels. Figure 5 illustrates carbon content and temperature relationship
for the AOD refining of carbon and low-alloy steels. Because the alloy content
of these grades of steel is substantially lower than that of stainless steel
and because the final carbon levels are generally higher, there is no
thermodynamic or practical reason for using an oxygen, inert-gas ratio of less
than 3:1.
Oxidation measurements indicate
that all of the oxygen reacts with the bath and that none leaves the vessel
unreacted. By monitoring and recording the oxygen consumption during refining,
very close control of end point carbon is achieved. Because the oxygen and
inert gases are introduced below the bath and at sonic velocities, there is
excellent bath mixing and intimate slag/metal contact. As a result, the
reaction kinetics of all chemical processes that take place within the vessel
are greatly improved.
Decarburization
In both stainless and low-alloy
steels, the dilution of oxygen with inert gas results in increased carbon
removal efficiencies without excessive metallic oxidation. In stainless grades,
carbon levels of 0.01% are readily obtained.
Chemistry Control
The excellent compositional control of AOD-refined steel is indicated in Table 2 for a ten-heat series of high-strength low-alloy steel. The injection of a known quantity of oxygen with a predetermined bath weight enables the steelmaker to obtain very tight chemical specifications.
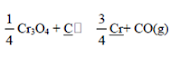






No comments:
Post a Comment